Here’s the translation into American English:
—
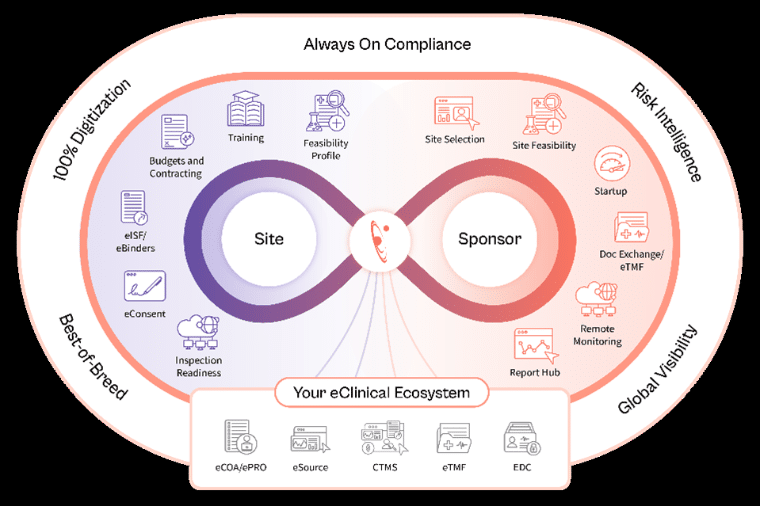
Florence Healthcare has taken a significant step in the clinical trial industry with the launch of its innovative Florence Trial Operations platform. This new tool connects 65,000 study sites with over 600 sponsors in more than 90 countries, establishing the company as the largest network in the sector and affirming its global leadership in clinical trial operations.
For the past six years, Florence has been recognized as the most efficient clinical trial technology, setting a standard for the speed of study initiation, automation of workflows, and management of operational risk within sponsors’ study portfolios. Ryan Jones, the company’s CEO, emphasized that “Florence was created to bring sponsors and sites together in a shared operational space.” He also highlighted that the company’s mission is to digitize study initiation to eliminate manual bottlenecks and paper workflows. The incorporation of artificial intelligence into the platform has improved the speed and quality of operations across the 65,000 study sites worldwide.
However, the clinical trial sector still faces considerable challenges. Currently, only 30% of sites worldwide use an electronic file management system, leaving nearly 200,000 sites to rely on inefficient paper processes. This lack of digitization leads to an estimated cost of one million dollars per study in terms of productivity and compliance.
Florence aims to close this digital gap through its research network, promoting the digitization of all operational workflows. As a result of these improvements, a 70% reduction in study initiation time and an annual savings of $141 million in operational costs have been observed. Additionally, there has been a notable increase in quality approval rates.
The company is integrating artificial intelligence across all stages of the study life cycle, enabling the identification and evaluation of research sites, optimizing recruitment, and facilitating remote monitoring. This ensures more effective and proactive oversight, minimizing the need for on-site interventions and guaranteeing that trials meet deadlines.
Florence will also showcase its new capabilities at the global Research Revolution 2025 event, which will be held from October 26 to 28, 2025. This event will bring together sponsors, CROs, and research sites, providing a space to explore these innovations live.
With its focus on improving workflows and fostering collaboration, Florence Healthcare is well-positioned to revolutionize the way clinical trials are conducted, promising a more agile and efficient future for medical research.
—
via: MiMub in Spanish